Received: Tue 23, May 2023
Accepted: Wed 14, Jun 2023
Abstract
Background: Robotic surgery has gain popularity in the past decades. We aim to investigate the impact of robotic surgery in gynecologic cancer. Methods: By retrospectively collecting demographics of those with gynecologic cancer performed by robotic surgery and addressing the perioperative and oncologic outcomes as the primary end points. Results: A total of 67 patients with 23 cervical cancer, 41 endometrial cancer. Amongst those with cervical cancer there were 15 (71.43%) international federation of gynecology and obstetrics (FIGO) stage 1 and 3 (14.29%) FIGO stage 2 with a median tumor size of 30.5 mm and a median 17 (0-42) lymph nodes retrieved with blood loss of 80 cc (5-500 cc). 6 recurrences (3 local/regional; 3 distant metastasis) with 5 succumb to disease with a 5-year disease-free and overall survival of 71.3%. In those with endometrial cancer there were 41 patients with 34 (85%) early stage (FIGO stage 1/2), 6 (15%) advanced stage (3/4). Median lymph nodes retrieval of 22 (0-60) and blood loss of 30 cc (1-2000 cc) were documented. There were 2 recurrences with 5 year disease-free and overall survival of 94.3% and 94.4% respectively. Conclusion: While robotic surgery has a superior surgical with comparable oncologic outcome in endometrial cancer, it should be used cautiously in unselected cervical cancer.
Keywords
Cervical cancer, endometrial cancer, minimal invasive surgery, radical hysterectomy, robotic assisted surgery
1. Introduction
Laparoscopy has gain acknowledgement and popularity in the treatment of gynecologic cancer with less blood loss, faster recovery, decrease perioperative morbidity and presumably comparable oncological results compared to conventional laparotomy in the past decades [1-5]. Robotic surgery, in the other hands have all the benefits of laparoscopy with added value of magnified surgical field, precision in complex procedure with ergonomic benefits for surgeons as well as a superior surgical with comparable oncological outcomes [6-11].
Minimal invasive surgery (MIS) (both laparoscopy and robotic) in the treatment of endometrial cancer has gain consensus amongst gynecologic oncologist [4, 5] and academic society like NCCN/ESGO have adopted its utility in the treatment of endometrial cancer. However, the utility of MIS in invasive cervical cancer has raised debate after the publication of the LACC study in 2018 [1], with higher recurrent rate and worst 4.5 years disease-free and overall survival in those receiving MIS as compared to conventional open radical hysterectomy.
Surgical volume, tumor size, uterine manipulator and intracorporeal colpotomy with insufflation during the MIS procedure were amongst the most prominent risk factors impacted the oncologic outcomes while performing radical hysterectomy in cervical cancer [1-3, 6-8]. We herein presented our preliminary surgical and oncologic outcomes after initiating the robotic surgery in 67 patients with gynecologic cancer treated in our institution from March 2013 to December 2020 with a median follow up of 45.8 months.
2. Materials and Methods
From March 2013 to December 2020, there were a total of 67 gynecologic cancer patients underwent robotic surgical procedure in our hospital with da Vinci (Si & Xi system, Intuitive surgical, Sunnyvale, Calif U.S.A). Amongst them, there were 23 cervical cancer (21 underwent hysterectomy/radical hysterectomy, 1 with synchronous endometrial cancer with postoperative radical parametrectomy after initial simple hysterectomy, 1 with paraaortic lymph node dissection before underwent definitive CCRT), 41 endometrial cancer (40 staging procedure with the same one having synchronous cervical cancer) and 3 ovarian cancer.
Most of the procedure were performed with spatula, ProGrasp, hot shear scissor, vessel sealer extend, mega needle drivers and force bipolar (intuitive surgical). Uterine manipulator (RUMI II Koh-Efficient –Uterine Manipulator CooperSurgical Trumbull, CT USA) were used in two cervical intraepithelial carcinomas (CIS),17 endometrial cancer and all of the ovarian cancer patients during the period with colpotomy made through intracorporeal approach. All lymph nodes were put inside an endo-bag before extraction through a 11 mm assistant port located at left lower quadrant and the uterine specimens delivered vaginally with the vaginal cuff close either through vaginally with a 1-0 vicryl suture or robotically with a barbed suture (V-Lock, Medtronic, Fridley, Minnesota U.S.A).
Sentinel lymph nodes mapping was not done in cervical cancer patients with only a handful early stage endometrial cancer underwent the mapping procedure during the study period. Demographic characteristics with clinico-pathological and surgical findings with intraoperative estimated blood loss (EBL), length of hospital stay (LOS) and major complications (bowels, urinary tracts, blood vessels injury, wound dehiscence, postoperative fever and ileus) as well as conversion rate were recorded. Operation time and console time were not presented in this study due to recall bias and inaccurate documentation.
Descriptive statistics were used to describe the demographic variables. Continuous variables were expressed as the median (range). Frequencies and their percentages were reported for categorical variables. The disease-specific overall survival and disease-free survival and were defined as the time from the initiation of treatment to the date of death from the diseases, and the time from treatment to first recurrence of the disease or death respectively. The patients died of other reason were censored. Survival outcomes were determined using the Kaplan-Meier method. The log-rank test was used to test for possible differences between different groups. P-value of < 0.05 was considered statistically significant. The study was approved by the appropriate institutional review board, and the requirement for written informed consent was waived by the institutional review board.
3. Results
A total of 23 cervical cancer (one with a bulky paraaortic lymph nodes receiving da vinci lymph node dissection before definitive CCRT and the other with incidental post simple hysterectomy finding of cervical IB1 tumor underwent radical parametrectomy were not included in the final analysis) entering the final analysis. There were 3 CIS, 15 (72%) FIGO stage 1, 3 (14%) stage II with 13 (62%) squamous cell carcinomas, 5 (24%) adenocarcinomas and 1 (5%) lymphoepithelioma-like and 1 (5%) neuroendocrine histology with a median age of 57 y/o (35-84) and BMI 25.08 kg/m2 (18.6-33.2). A median tumor diameter of 30.5 mm (2-73mm) and a median 17 (0-42) of lymph node retrieved with an estimated blood loss of 80 cc (5-500 cc) and length of hospital stay of 6 days (2-9 days) were noted (Table 1).
TABLE 1: Demographic characteristics.
|
Parameter |
Cervical (n=21) |
Endometrial (n=40) |
Ovarian (n=3) |
|
Age (years) |
57 (35- 84) |
56 (42- 75) |
48 (43- 59) |
|
Height (cm) |
154 (148- 165) |
154 (142- 167) |
154 (152- 155) |
|
Weight (kg) |
63 (45- 83) |
62.5 (43- 94) |
54 (49- 57) |
|
BMI (kg/m^2) |
25.08 (18.59- 33.25) |
26.59 (19.05- 37.18) |
23.37 (20.4- 24.03) |
|
LN retrieve |
17 (0- 42) |
22 (0- 60) |
12 (8- 32) |
|
Length of Hospital Stay (LOH) |
6 (2- 9) |
4 (2- 83) |
4 (3- 5) |
|
Estimated Blood loss (EBL)(cc) |
80 (5- 500) |
30 (1- 2000) |
100 (50- 200) |
|
Tumor size (mm) |
30.5 (2- 73) |
35 (3- 151) |
79 (23- 135) |
|
Histology |
|
|
|
|
adneocarcinoma |
5 (23.82) |
0 (0) |
0 (0) |
|
carcinoma in situ |
1 (4.76) |
0 (0) |
0 (0) |
|
Lymphoepithelioma like |
1 (4.76) |
0 (0) |
0 (0) |
|
neuroendocrine |
1 (4.76) |
0 (0) |
0 (0) |
|
Squamous cell carcinoma |
13 (61.9) |
0 (0) |
0 (0) |
|
adenosquamous cell carcinoma |
0 (0) |
1 (2.5) |
0 (0) |
|
endometrioid |
0 (0) |
33 (82.5) |
0 (0) |
|
mixed |
0 (0) |
3 (7.5) |
1 (33.34) |
|
serous |
0 (0) |
2 (5) |
0 (0) |
|
carcinosarcoma |
|
1 (2.5) |
|
|
adult granulosa cell tumor |
0 (0) |
0 (0) |
1 (33.33) |
|
clear cell |
0 (0) |
0 (0) |
0 (0) |
|
mucinous |
0 (0) |
0 (0) |
1 (33.33) |
|
Grading |
|
|
|
|
1 |
2 (9.52) |
11 (27.5) |
2 (66.67) |
|
2 |
14 (66.67) |
17 (42.5) |
1 (33.33) |
|
3 |
2 (9.52) |
12 (30) |
0 (0) |
|
AJCC stage |
|
|
|
|
0 |
2 (9.52) |
2 (5) |
0 (0) |
|
1 |
12 (57.14) |
31 (77.5) |
3 (100) |
|
2 |
3 (14.29) |
1 (2.5) |
0 (0) |
|
3 |
2 (9.52) |
6 (15) |
0 (0) |
|
4 |
1 (4.76) |
0 (0) |
0 (0) |
|
FIGO stage |
|
|
|
|
0 |
3 (14.29) |
2 (5) |
0 (0) |
|
1 |
15 (71.42) |
31 (77.5) |
3 (100) |
|
2 |
3 (14.29) |
1 (2.5) |
0 (0) |
|
3 |
0 (0) |
6 (15) |
0 (0) |
|
4 |
0 (0) |
0 (0) |
0 (0) |
|
All- cause mortality |
6 (28.57) |
3 (7.5) |
0 (0) |
|
Recurrence |
6 (28.57) |
2 (5) |
0 (0) |
|
Recurrent pattern |
|
|
|
|
Local/Regional |
4 (19.05) |
1 (2.5) |
0 (0) |
|
Distant |
2 (9.52) |
1 (2.5) |
0 (0) |
|
Major Complications |
1 (4.76) |
2 (5) |
0 (0) |
|
Disease-specific mortality |
5 (23.81) |
2 (5) |
0(0) |
|
Risk factor |
|
|
|
|
LN+ |
1 (4.76) |
2 (5) |
0 (0) |
|
LVSI+ |
3 (14.29) |
3 (7.5) |
0 (0) |
|
LN+ & LVSI+ |
1 (4.76) |
1 (2.5) |
0 (0) |
|
LVSI+ parametrium+ |
1 (4.76) |
0 (0) |
0 (0) |
|
parametrium+ |
1 (4.76) |
1 (2.5) |
0 (0) |
|
Adjuvant Therapy |
|
|
|
|
C/T |
0(0) |
6 (15) |
1 (33.33) |
|
R/T |
2 (9.52) |
10 (25) |
0(0) |
|
CCRT |
5 (23.81) |
0 (0) |
0 (0) |
|
Sequential |
0(0) |
6 (15) |
0(0) |
|
Manipulator used |
2 (9.52) |
17(41.46) |
0 (0) |
|
Conversion rate |
0 |
0 |
0 |
|
Data are presented as median (range) or number
(%) |
|||
7/21 (33%) receiving post -operative adjuvant treatment due to either close surgical margins, lymph nodes metastasis, positive parametrium or lymphovascular space invasion with 5 of them receiving CCRT and 2 RT (Table 1). Amongst those treated with robotic radical hysterectomy, recurrence occurs in six, with 2 of them having AJCC stage 1B2, 2 with stage II, 1 with stage III and 1 with stage IV, with an average tumor size of 45 mm (30 mm- 73 mm) as compare to an average of 25 mm in those didn’t experience recurrence (Table 2). There were 3 (50%) local/regional recurrence with 3 (50%) distant metastasis with four of them receiving palliative C/T, 2 with surgical resection resultant in five patients succumbed to their disease (84%) except one who was salvage successfully with anterior pelvic exenteration with no evidence of disease at the time of follow up (Table 2).
TABLE 2: Characteristic of recurrent cervical cancer (201303-202003).
|
Year |
Recurrence/ Number Operated |
stage AJCC |
Risk factor |
Size |
Adjuvant Therapy |
Recurrence Interval(months) |
Site |
salvage |
Status |
|
2013 |
2/3 |
IIa |
Nil |
35 |
Nil |
76 |
L/R |
Exenteration |
NED |
|
2013 |
|
III |
LN+LVSI+ |
30 |
CCRT |
23 |
Distant |
C/T |
DOD |
|
2014 |
1/1 |
IV |
neuroendocrine |
36 |
CCRT |
persisted diseases |
Both |
C/T |
DOD |
|
2015 |
1/7 |
Ib2 |
LVSI/bulky |
73 |
R/T |
5 |
L/R |
C/T |
DOD |
|
2016 |
1/4 |
II |
LVSI+Parametrium+ |
50 |
CCRT |
33 |
Distant |
C/T |
DOD |
|
2018 |
1/2 |
Ib2 |
LVSI |
45 |
R/T |
7 |
L/R |
surgery |
DOD |
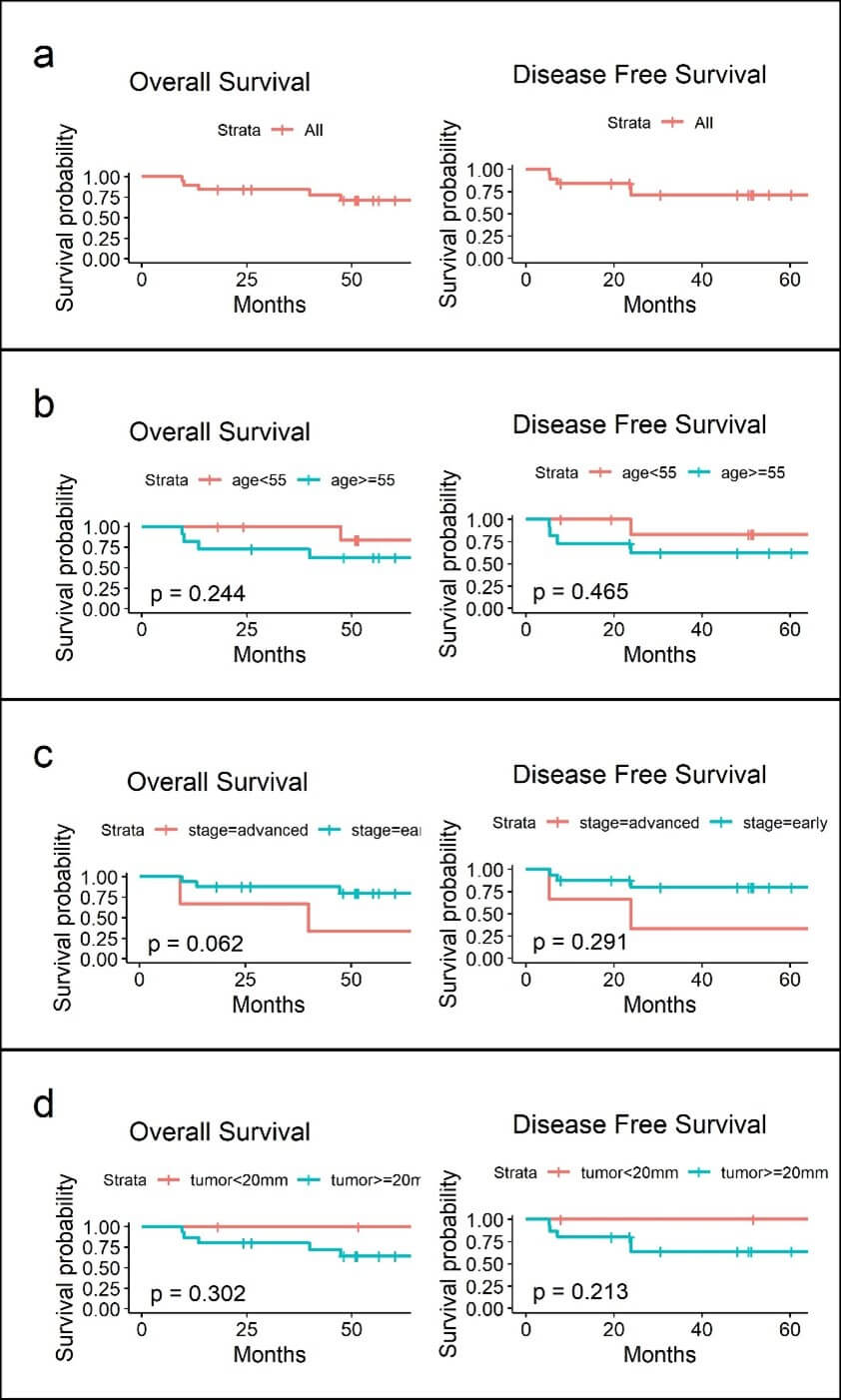
Table 3 depict the all-cause mortality of the 6 patients with all, but one died of advance colon cancer. In 5 of those with cervical cancer recurrence 3 received chemotherapy, 1 with CCRT and 2 surgical resections with all succumb to their disease with an average survival months of 10.2 months after the salvage treatment. A 5 year disease-free and overall survival of 71.3% was noted (Figure 1a) which was not related to age, AJCC staging or tumor size (Figures 1b-1d).
TABLE 3: All-cause mortality.
|
All- cause mortality |
salvage therapy |
recurrent to death |
|
1 cervical cancer |
surgery (rectal recurrence) |
6 months |
|
2 cervical cancer |
C/T |
16 months |
|
3 cervical cancer |
CCRT |
4 months |
|
4 cervical cancer |
C/T |
12 months |
|
5 colon cancer |
Surgery |
|
|
6 cervical cancer |
C/T |
13 months |
During the same period ,there were a total of 41 endometrial cancer (one with synchronous cervical cancer receiving postoperative radical parametrectomy was not included in the final analysis) with 33 (82.5%) endometrioid, 3 (7.5%) mixed type, 2 (5%) serous and 1 carcinosarcoms (2.5%), with FIGO stage I/II 34 (77.5%) and advance stage (III/IV) of 6 (15%), tumor differentiation grade 1 11 (27.5%) grade-2 17 (42.5%) and grade-3 12 (30 %).
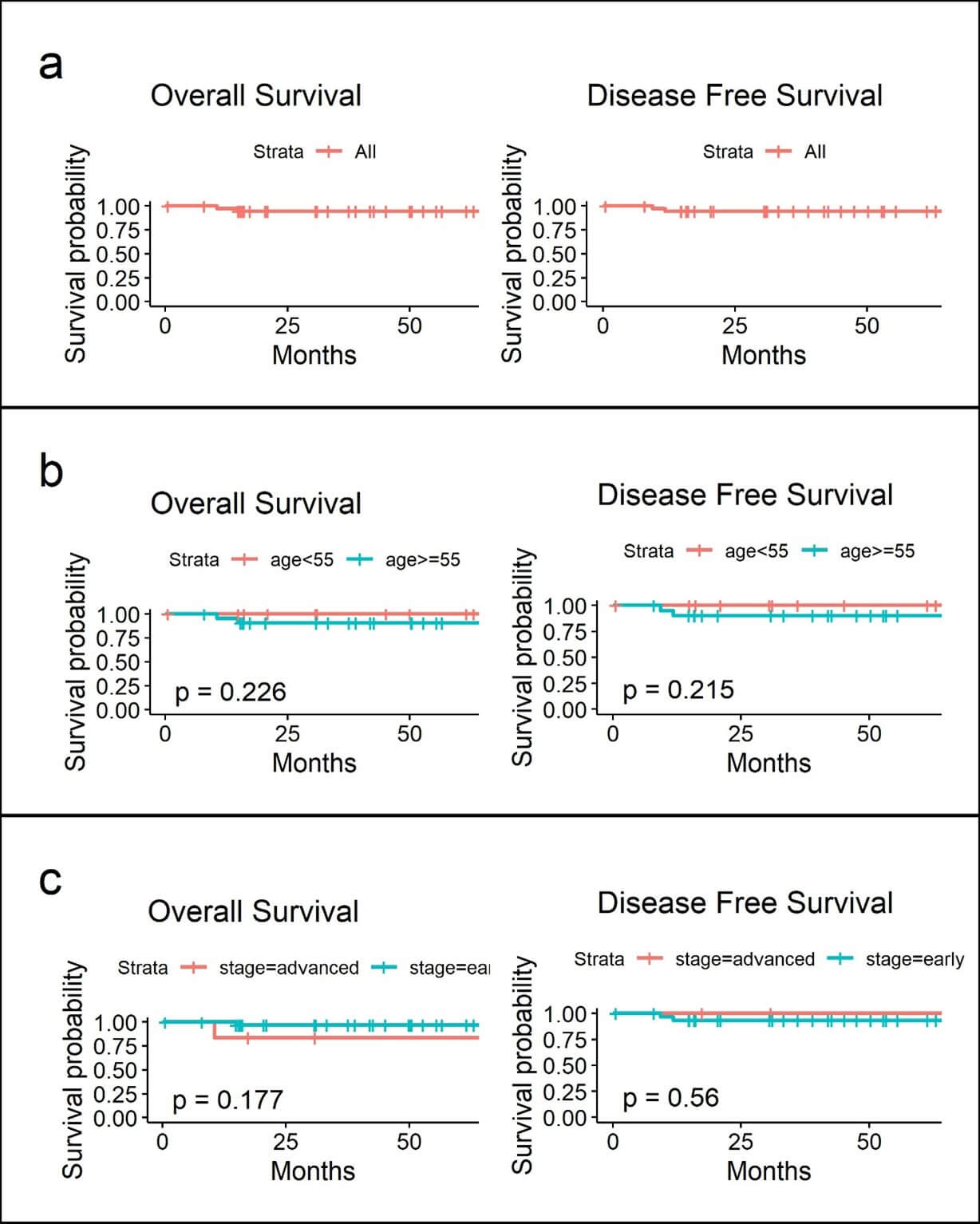
Postoperative adjuvant therapy was given in 22 patients with 6 receiving C/T, 10 RT and 14 sequential C/T and R/T (sandwich therapy) with 2 recurrence (5%) during the follow up, one over vaginal cuff (manipulator used) salvage with radiation and one with distant metastasis. There were 2 deaths resulting in a 5 years disease-free and overall survival rate of 94.3 and 94.4 % respectively (Table 1 & Figure 2a). There were a total of 3 patients with early stage ovarian cancer with one mucinous, one adult type granulosa cell and one mixed type histology in the ovarian cancer groups with no recurrence or death occur.
Median lymph node retrieve was 17 (0-42), 22 (0-60) and 12 (8-32) respectively in cervical, endometrial and ovarian cancer with an estimated blood loss of 80 cc (5-500), 30 cc (1-2000), 100 cc (50-200) respectively in cervical, endometrial and ovarian cancer. One massive bleeding of 500 cc in the cervical cohort and another with 2000 cc in endometrial cancer group due to inadvertent great vessel injury (Table 1). Median length of hospital stay were 6 (cervical cancer), 4 (endometrial cancer) and 4 (ovarian cancer) in our series.
No a single conversion to laparotomy was recorded in these 67 procedures with 1 postoperative fever, two massive blood loss and one cerebral insufficiency were noted with a major complication rate of 4. 8% and 5% respectively in the cervical and endometrial cancer group.
4. Discussion
4.1. Main Findings
5 years disease- specific and overall survival in cervical cancer and endometrial cancer treated with robotic procedure in our institution were 71.3 % and 94.3 %/ 94.4% respectively in this cohort study, with exceedingly high recurrence (28.6%) and disease specific mortality rate (24%) in cervical cancers patients. On the contrary, the favorable survival and perioperative outcomes seen in the endometrial cancer cohort was consistent with previous studies.
4.2. Interpretation of Findings
The average size of tumor in those with recurrence in cervical cancer in our series was larger (45mm) compare to those who did not experienced recurrence (25mm), adding the potential risk pertinent to recurrence such as a more invasive histology and lymph vascular space invasion were also noted in the same cohort. Although not a detrimental confounding factor in survival analysis, a presumably greater risk in larger tumor even without the usage of a uterine manipulator should be taken into consideration because all of the colpotomy in our series were done intracorporeally, which could lead to tumor spillage / seeding resulting in distant metastasis as also mentioned in other studies [1-3, 6-8].
Surgical volume and treatment centralization probably is another issue should be addressed for, especially in radical hysterectomy as reported in the article by Alfonzo et al and others [12, 13] which has seen no difference in survival in patients treating with robotic surgery versus those with open approaches. This was not practical in our country due to steadily decreasing incidence of invasive cervical cancer. Other protective measures such as vaginal closure of tumor and/or preoperative conization in tumor less than 2cm should be excised to further improving the oncological outcomes.
As contrary to cervical cancer and in parallel with other previous confirmatory reports, our study found that robotic surgery in early-stage endometrial cancer has an excellent surgical as well as oncologic outcomes as compare to laparotomy with a 5 year disease-specific and overall survival of 94.3 and 94.4% respectively in our study. One recurrence over vaginal cuff was noted albeit there were 17 of them using uterine manipulators, and was salvageable with radiation which probably implying a less contributory risks posed on early endometrial cancer as compare to a “exposed” cervical tumor.
The adequacy of lymph nodes retrieved in our study emphasizing the feasibility and excellent performance of robotic surgery in lymphadenectomy. The concept of sentinel lymph node mapping was apply only in a handful endometrial cancer in a later years in our study and should be incorporate in the future planning for saving operating times as well as decreasing postoperative morbidity following a more comprehensive lymphadenectomy.
Another remarkable finding in our study is the zero-conversion rate to laparotomy in all 67 procedures with minimal blood loss and few postoperative complications, implying the potential benefits of laparoscopy proficiency in a surgeon before ‘jumping” into the utility of robotic surgery specifically in those with complicated gynecological cancer procedure. This is the first series of systemic retrospective analysis of robotic surgery in gynecology cancers in Taiwan, hopefully our experiences could provide benefits for those proposing their exploration in the utility of robotic in gynecologic cancer.
A strong and cohesive operating team play a pivotal role in order to have a smooth surgical procedure. Analyses of surgical and oncologic outcomes from other institutions is greatly anticipated especially in surgeon performing the robotic radical hysterectomy in carefully selected patients in the near futures.
5. Conclusion
While robotic surgery shows a superior surgical outcome with comparable oncologic outcomes in endometrial cancer, it should be used cautiously in cervical cancer.
Strengths and Limitations
The strengths of this study is its consistency with all the surgical procedure performed by one surgeon who are familiar with gynecologic oncology surgical procedure and accurate documentation of all the demographics in our patients. The limitations invariably lie in its small sample size and inherent retrospective context, which give a generalizable recommendation impossible and lack of comparable cohort at the same period.
Acknowledgments
None.
Funding
None.
Conflicts of Interest
None.
IRB Number
111006.
REFERENCES
[1] Pedro T Ramirez,
Michael Frumovitz, Rene Pareja, et al. “Minimally Invasive versus Abdominal
Radical Hysterectomy for Cervical Cancer.” N
Engl J Med, vol. 379, no. 20, pp. 1895-1904, 2018. View at: Publisher Site | PubMed
[2] Benny Brandt,
Vasileios Sioulas, Derman Basaran, et al. “Minimally invasive surgery versus
laparotomy for radical hysterectomy in the management of early-stage cervical
cancer: Survival outcomes.” Gynecol Oncol,
vol. 156, no. 3, pp.
591-597, 2020. View at: Publisher
Site | PubMed
[3] Sha-Sha Zhang, Tian
Ding, Zheng-Hui Cui, et al. “Efficacy of robotic radical hysterectomy for
cervical cancer compared with that of open and laparoscopic surgery: A separate
meta-analysis of high-quality studies.” Medicine
(Baltimore), vol. 98, no.
4, pp. e14171, 2019. View at: Publisher Site | PubMed
[4] Joan L Walker,
Marion R Piedmonte, Nick M Spirtos, et al. “Laparoscopy compared with
laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic
Oncology Group Study LAP2.” J Clin Oncol,
vol. 27, no. 32, pp. 5331-5336, 2009. View at: Publisher Site | PubMed
[5] Amy J Bregar,
Alexander Melamed, Elisabeth Diver, et al. “Minimally Invasive Staging Surgery
in Women with Early-Stage Endometrial Cancer: Analysis of the National Cancer
Data Base.” Ann Surg Oncolo, vol. 24, no. 6, pp. 1677-1687,
2017. View at: Publisher
Site | PubMed
[6] Tiefeng Cao,
Yanling Feng, Qidan Huang, et al. “Prognostic and Safety Roles in Laparoscopic
Versus Abdominal Radical Hysterectomy in Cervical Cancer: A Meta-analysis.” J Laparendosc Adv Surg Tech A, vol. 25,
no. 12, pp. 990-998, 2015. View at: Publisher Site | PubMed
[7] B M Sert, J F
Boggess, S Ahmad, et al. “Robot-assisted versus open radical hysterectomy: a
multi-institutional experience for early-stage cervical cancer.” Eur J Surg Oncol, vol. 42, no. 4, pp.
513-522, 2016. View at: Publisher
Site | PubMed
[8] Chirag A Shah,
Tiffany Beck, John B Liao, et al. “Surgical and oncologic outcomes after
robotic radical hysterectomy as compared to open radical hysterectomy in the
treatment of early cervical cancer.” J
Gynecol Oncol, vol. 28, no. 6, pp. e82, 2017. View at: Publisher Site | PubMed
[9] Tae-Wook Kong,
Suk-Joon Chang, Xianling Piao, et al. “Patterns of recurrence and survival
after abdominal versus laparoscopic/robotic radical hysterectomy in patients
with early cervical cancer.” J Obstet
Gynaecol Res, vol. 42, no. 1, pp. 77-86, 2016. View at: Publisher Site | PubMed
[10]
Dimitrios
Nasioudis, Maureen Byrne, Emily M Ko, et al. “Minimally invasive hysterectomy
for stage IA cervical carcinoma: a survival analysis of the National Cancer
Database.” Int J Gynecol Cancer, vol.
31, no. 8, pp. 1099-103, 2021. View at: Publisher Site | PubMed
[11]
Roni
Nitecki, Pedro T Ramirez, Michael Frumovitz, et al. “Survival After Minimally
Invasive vs Open Radical Hysterectomy for Early-Stage Cervical Cancer: A
Systematic Review and Meta-analysis.” JAMA
Oncol. vol. 6, no. 7, pp. 1019-1027, 2020. View at: Publisher Site | PubMed
[12] Koji Matsuo, Muneaki Shimada, Satoshi Yamaguchi, et al. “Association of Radical Hysterectomy Surgical Volume and Survival for Early-Stage Cervical Cancer.” Obstet Gynecol, vol. 133, no. 6, pp. 1086-1098, 2019. View at: Publisher Site | PubMed
[13] Emilia Alfonzo, Emelie Wallin, Linnea Ekdahl, et al. “No survival difference between robotic and open radical hysterectomy for women with early-stage cervical cancer: results from a nationwide population-based cohort study.” Eur J Cancer, vol. 116, pp. 169-177, 2019. View at: Publisher Site | PubMed
