Received: Sat 10, Jun 2023
Accepted: Tue 27, Jun 2023
Abstract
Background: Recent studies have reported higher postoperative complication rates in patients with severe obesity who undergo bariatric surgery. The extremely obese patient deserves special consideration: significant comorbidities, technical difficulties, and increased postoperative morbidity and mortality are all expected in this patient population. Current data are limited and discrepant on the relationship between patients with class IV obesity (body mass index (BMI) ≥50-59.9 kg/m2), and class V obesity (BMI ≥60 kg/m2). This study compared early postoperative complications (≤30-day) following one-anastomosis gastric bypass (OAGB) morbidity in patients with class III, IV, and V obesity. Methods: Retrospective analysis of perioperative OAGB outcomes in three BMI groups at a high-volume hospital. Operative time, length of stay (LOS), and overall early postoperative complication rates were studied. Complications were ranked by Clavien-Dindo classification (CDC). Results: Between January 2017-December 2021, consecutive patients with obesity class III (n= 2,950), IV (n= 256), and V (n= 23) underwent OAGB. BMI groups were comparable in gender, age, and associated comorbidities. Mean operative time was significantly longer in the higher BMI groups: class III (66.5±25.6 min), IV (70.5±28.7 min), and V (80.0±34.7 min), respectively (p= 0.018); no difference in LOS. In respective BMI classes, ≤30-day complication rates were 3.2%, 3.5%, and 4.3% (p= 0.926). The respective number of patients with CDC grades of 1-2 were 45 (1.5%), 6 (2.3%), and 1 (4.3%), p= 0.500; and in grade ≥3a, 25 (0.8%), 1 (0.4%), 0 (0.0%), p= 0.669. No significant differences in rates of early complications, reoperations, and readmissions were found in revisional patients across BMI groups. There was 0.06% mortality (n= 2 in 3,229), both in BMI class III. Conclusion: OAGB is a safe metabolic bariatric surgery procedure in patients with class III, IV, and V obesity in the perioperative term with comparable ≤30-day morbidity in the three BMI groups.
Keywords
Bariatric Surgery, early postoperative complications, class III obesity, class IV obesity, class V obesity, one anastomosis gastric bypass
1. Introduction
Class III obesity is defined as body mass index (BMI) ≥ 40-49.9 kg/m2, Class IV obesity as BMI ≥ 50-59.9 kg/m2, and Class V as BMI ≥ 60 kg/m2. The prevalence of higher obesity classes is increasing [1, 2]. Patients with class IV and class V obesity suffer greatly from associated comorbidities (e.g., type 2 diabetes, hypertension, hyperlipidemia, obstructive sleep apnea). They may benefit from metabolic bariatric surgeries (MBS), yet their higher BMIs may also be associated with increased perioperative risks [3-7]. Significant comorbidities, technical difficulties, and increased postoperative morbidity and mortality are all expected in the extremely obese patient population.
Performing any MBS procedure in patients with class IV and V obesity can be challenging, especially due to the thickening of the abdominal wall and abundant adipose tissue. The one-anastomosis gastric bypass (OAGB) procedure first reported by Rutledge et al. in 1997 [8, 9] has gradually gained wide acceptance and was recently endorsed by the American Society for Metabolic and Bariatric Surgery (ASMBS) and the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) [10]. Several studies have reported OAGB outcomes in high-BMI cohorts and found comparable 30-day safety results relative to sleeve gastrectomy (SG) [11-14]. In addition, a systematic review comparing OAGB, Roux-en-Y gastric bypass (RYGB), and sleeve gastrectomy (SG) concluded that OAGB was an equally safe and effective operation for managing patients in high BMI groups [15].
The current study of early OAGB outcomes was conducted at a high-volume bariatric center in Israel [16]. A survey by the Israel Central Bureau of Statistics conducted in 2017 found that 48% of the Israeli population above the age of 20 were overweight or obese [17]. Analysis of trends in MBS in Israel found that over the last several years, the most commonly performed MBS procedure shifted from SG to OAGB [5]. The aim of this study was to evaluate the perioperative safety of OAGB in patients with class IV and V obesity in comparison with those with class III obesity.
2. Methods
2.1. Study Design
Retrospective cohort study utilizing data that was retrieved from electronic medical records. Data collected included pre-, peri-, and 30-day postoperative outcomes of 3299 primary and revisional OAGB procedures that were conducted at the Assuta Bariatric Centers (ABCs). Patients with class IV or V obesity were compared to those with class III looking at baseline characteristics, operative factors, and ≤30-day postoperative complications. The study protocol was approved by the institutional review board (43-20-ASMC 13/9/2020). Informed consent was waived due to the retrospective and anonymous nature of data collected. All patients above the age of 18 years who underwent OAGB between January 1, 2017 and December 31, 2021 and had class III, IV, or V obesity were included.
2.2. Study Procedure and Outcomes
All operative data (operative time, laparoscopic or other approaches, length of stay in hospital [LOS], prior MBS procedures, additional procedures) and operative follow-up were recorded by the multidisciplinary surgical team into patients’ electronic medical records as were complications during the first 30 postoperative days. Complications were categorized according to the Clavien-Dindo classification (CDC) [18]. Complications ranked as a CDC grade 1-2 was regarded as minor, while a CDC grade ≥3a-5 were termed as major events. The standardized surgical technique and postoperative care regimen for OAGB used in our center have been detailed previously [19].
2.3. Statistical Analysis
Analyses were performed using the IBM SPSS statistical package (version 28.0, SPSS Inc, IBM Corp., Armonk, NY). Continuous variables were presented as means and standard deviations (SD), while categorical variables were presented as frequencies and percentages. Data normality was assessed using the Kolmogorov-Smirnov test. Between group differences were analyzed using the Kruskal-Wallis test, Chi-square test, or Fisher’s exact test, as appropriate. All statistical tests were two-tailed, and statistical significance was set at p<0.05. For multiple comparisons, a false detection rate (FDR) <0.1 was considered significant.
3. Results
3.1. Patient Characteristics
Between January 1, 2017, and December 31, 2021, eligible 3,229 patients underwent OAGB, and completed their 30-day follow-up. Mean age was 39.0±11.8 years, and 2,361(73.1%) were women. Mean preoperative BMI was 44.2±4.0 kg/m2. The most common obesity-related comorbidity was hyperlipidemia (1,137; 35.2%), followed by type2 diabetes mellitus (T2DM) (824; 25.5%). OAGB was performed as revisional MBS in 687 (21.3%) patients, and an additional procedure was performed in 623 (19.3%) patients. The total early (<30 days) postoperative complication rate was 3.2%. Most of the patients were classified as class III obesity (2,950; 91.4%), followed by IV (256; 7.9%) and V (23; 0.7%) obesity. There were no differences between these three groups with regard to baseline patients' characteristics, except BMI (43.3±2.6, 53.3±2.5, and 62.23±1.9 kg/m2 in patients with class III, IV, and V obesity, respectively; p<0.001) (Table 1).
3.2. Surgical Characteristics
The mean operative time was significantly longer as BMI increased. Respective mean operative time in class III, IV, and V was 66.5±25.6, 70.5±28.7, and 80.0±34.7 minutes; p=0.018. Length of hospitalization was similar across groups with no statistically significant difference. The most common previous MBS procedure was laparoscopic adjustable gastric banding (LAGB), followed by SG. In respective class III, IV, and V groups, 465 (15.8%), 37 (14.5%), and 5 (2.7%) patients had a history of previous LAGB (p=0.624). Laparoscopic cholecystectomy, performed in 392 (12.1%) patients, was the most common additional procedure, followed by repair of diaphragmatic (hiatal) hernia in 196 (6.0%) patients. There was no statistical difference between the three study groups in terms of previous MBS type or additional procedures (Table 2).
TABLE 1: Patient characteristics.
|
Obesity
Class III (n=2,950) |
Obesity
Class IV (n=256) |
Obesity
Class V (n=23) |
P-value |
|
|
Age
(years) mean ± SD |
39.0±11.7 |
38.5±12.5 |
42.5±13.9 |
0.329 |
|
Females,
n (%) |
2,173
(73.7) |
171
(66.9) |
17
(73.9) |
0.059 |
|
Preop.
BMI (kg/m2) mean ± SD |
43.3±2.6 |
53.3±2.5 |
62.23±1.9 |
<0.001 |
|
Smoker,
n (%) |
181
(6.1) |
20
(7.8) |
2
(8.7) |
0.509 |
|
Comorbidities |
|
|
|
|
|
·
Type 2 diabetes mellitus, n (%) |
766
(26.0) |
51
(19.9) |
7
(30.4) |
0.090 |
|
·
Hypertension, n (%) |
555
(18.8) |
47
(18.4) |
8
(34.8) |
0.146 |
|
·
Dyslipidemia, n (%) |
1046
(35.5) |
83
(32.4) |
8
(34.8) |
0.621 |
|
·
Fatty liver (NAFLD), n (%) |
2,024
(68.6) |
173
(67.6) |
18
(78.3) |
0.571 |
|
·
OSA, n (%) |
210
(7.1) |
25
(9.8) |
3
(13) |
0.119 |
Obesity
Class III, body mass index [BMI] ≥40-49.9 kg/m2; Obesity Class IV,
BMI ≥50-59.9 kg/m2; Obesity Class V, BMI ≥60 kg/m2.
Preop: Preoperative; BMI: Body
Mass Index; NAFLD: Non-alcoholic Fatty Liver Disease; OSA: Obstructive Sleep
Apnea; SD: Standard Deviation.
TABLE 2: Surgical Characteristics.
|
Obesity
Class III (n=2,950) |
Obesity
Class IV (n=256) |
Obesity
Class V (n=23) |
P-value |
|
|
Operative
length (minutes) mean±SD
(median) |
66.5±25.6
(61.0) |
70.5±28.7
(64.0) |
80.0±34.7
(62.0) |
0.018 |
|
Laparoscopic
approach, n (%) |
2,948
(99.9) |
254
(99.6) |
23
(100.0) |
>0.999 |
|
Length
of hospitalization (days) mean±SD (median, range) |
2.2±1.1 (2.0
,1.9-2.2) |
2.4±1.9 (2.0
,1.9-2.2) |
2.3±0.4 (2.1
,1.9-2.6) |
0.131 |
|
Previous
Bariatric surgery, n (%) |
638
(21.6) |
44
(17.2) |
5
(21.7) |
0.250 |
|
·
LAGB, n (%) |
465
(15.8) |
37
(14.5) |
5
(2.7) |
0.624 |
|
·
LSG, n (%) |
185
(6.3) |
11
(4.3) |
0
(0.0) |
0.211 |
|
·
VBG, n (%) |
37
(1.3) |
5
(2.0) |
0
(0.0) |
0.548 |
|
Additional
Procedure, n (%) |
573
(19.4) |
48
(18.8) |
2
(8.7) |
0.419 |
|
·
Diaphragmatic hernia repair1, n (%) |
176
(6.0) |
19
(7.4) |
1
(4.3) |
0.608 |
|
·
Cholecystectomy1, n (%) |
364
(12.3) |
27
(10.5) |
1
(4.3) |
0.363 |
|
·
Partial gastrectomy1, n (%) |
45
(1.5) |
5
(2.0) |
0
(0.0) |
0.724 |
|
·
Ventral hernia repair1, n (%) |
30
(1.0) |
3
(1.2) |
0
(0.0) |
0.863 |
Obesity
Class III, body mass index [BMI] ≥40-49.9 kg/m2; Obesity Class IV
obesity, BMI ≥50-59.9 kg/m2; Obesity Class V obesity, BMI ≥60 kg/m2.
SD:
Standard Deviation; LAGB: Laparoscopic Adjustable Gastric Banding; LSG:
Laparoscopic Sleeve Gastrectomy; VGB: Vertical Banded Gastroplasty.
1Additional
procedures during the surgery - could be more than one additional procedure in
one surgery.
3.3. Surgical Adverse Events
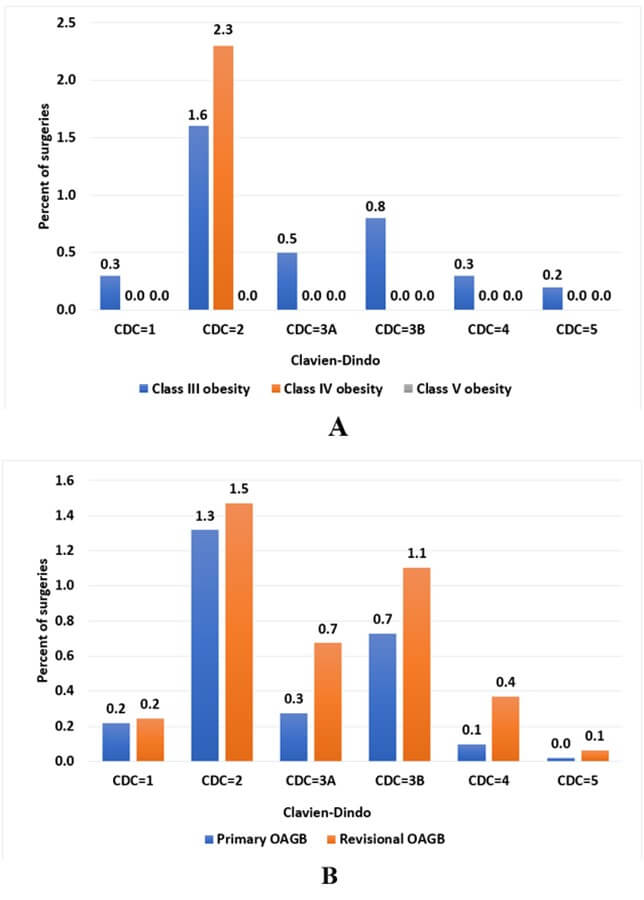
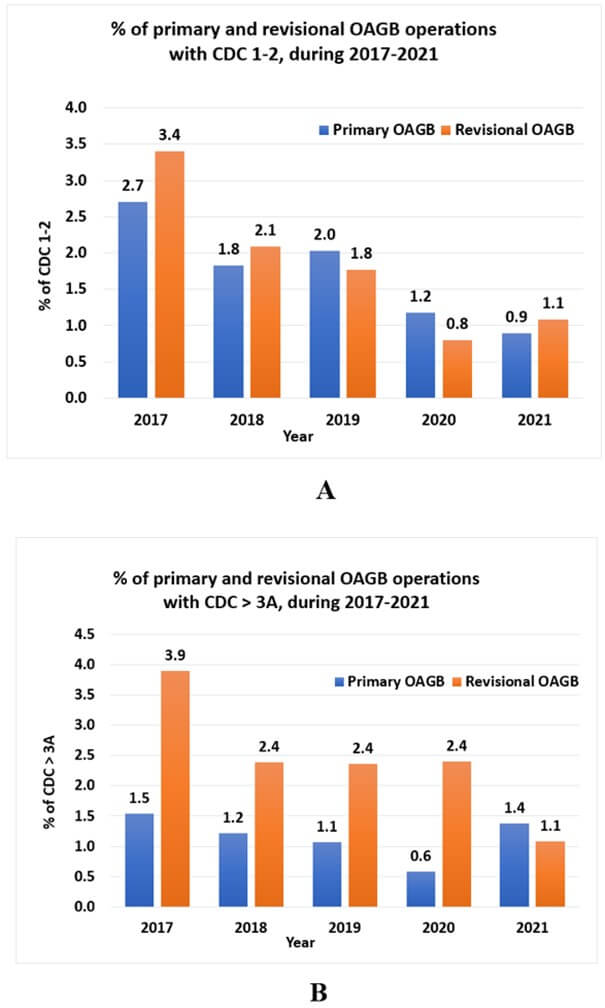
The respective early postoperative complication rate (≤ 30 days) was comparable between study groups (95 [3.2%], 9 [3.5%], 1 [4.3%]; p=0.926) (Table 3). The percentages of total complications by CDC grade, class III, IV, and V groups, and primary and revisional OAGB are presented in (Figure 1). CDC 2 is the most frequent grade, with higher presentation among class IV obese patients (2.3%) compared to class III (1.6%), and among revisional OAGB (1.4%) compared to primary OAGB (1.3%) as well. The distribution of minor complications (CDC grade ≤ 2) and major complications (CDC grade ≥3a-5) over the study period is presented in (Figure 2) by primary and revisional OAGB. There is a decreasing trend in minor and major complications over the study period.
TABLE 3: 30-Days postoperative adverse events.
|
Class
III obesity |
Class
IV obesity |
Class V obesity |
P-value |
||||
|
|
Total
(n=2950) |
Revisional
(n=638) |
Total
(n=256) |
Revisional
(n=44) |
Total
(n=23) |
Revisional
(n=5) |
Total/ Revisional |
|
Total
Early Complication (<30 Day), n (%) |
95
(3.2) |
29
(4.5) |
9
(3.5) |
2(4.5) |
1(4.3) |
0
(0.0) |
0.926/0.888 |
|
·
Bleeding, n (%) |
51
(1.7) |
14
(2.2) |
5
(2.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.787/0.578 |
|
·
Leak, n (%) |
10
(0.3) |
5
(0.8) |
1
(0.4) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.952/0.824 |
|
·
Obstruction, n (%) |
2
(0.1) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.910/NA |
|
·
Small bowel injury, n (%) |
1
(0.03) |
1
(0.2) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.954/0.962 |
|
·
Respiratory complications, n (%) |
6
(0.2) |
3
(0.5) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.753/0.891 |
|
·
Infection, n (%) |
4
(0.1) |
1
(0.2) |
1
(0.4) |
1
(2.3) |
0
(0.0) |
0
(0.0) |
0.598/0.042 |
|
·
Acute renal failure, n (%) |
3
(0.1) |
2
(0.3) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.868/0.926 |
|
CDC≤2,
n (%) |
45
(1.5) |
12
(1.9) |
6
(2.3) |
1
(2.3) |
1 (4.3) |
0
(0.0) |
0.500/0.937 |
|
CDC≥3A,
n (%) |
25
(0.8) |
11
(1.7) |
1
(0.4) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.669/0.651 |
|
Early
readmission (<30 days), n (%) |
47
(1.6) |
15
(2.4) |
4
(1.6) |
2
(4.5) |
1
(4.3) |
0
(0.0) |
0.568/0.622 |
|
Early
reoperations (<30 days), n (%) |
17
(0.6) |
5
(0.8) |
1
(0.4) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.871/0.824 |
|
30-day
mortality rate, n (%) |
2
(0.06) |
1
(0.16) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0.910/0.962 |
FDR (false discovery rate) > 0.950 for all of the
above comparisons.
Obesity Class III, body mass index [BMI] ≥40-49.9
kg/m2; Obesity Class IV, BMI ≥50-59.9 kg/m2; Obesity
Class V, BMI ≥60 kg/m2.
SD: Standard Deviation; CDC: Clavien-Dindo
Classification; NA: Not applicable.
The most common adverse event was bleeding (56; 1.7%). In class III and IV, respectively, 28 and 4 cases of extra-luminal bleeding and 23 and 1 cases of intra-luminal bleeding were identified. There were no cases of bleeding in the class V group. There was no statistical difference between the three obesity groups in term of early postoperative complications, including early readmission and reoperation, as well as the postoperative intensive care unit admissions (Table 3). The most common reason for reoperation was bleeding, followed by leak. We identified 8 cases of reoperation due to bleeding and 7 cases due to leak in the class III group. There was only one case of reoperation in the class IV group due to bleeding from the staple line. There were no reoperations in the class V group.
Similarly, the rate of early complications, reoperations, and readmissions post revisional OAGB surgeries were similar in all three BMI groups (Table 3). Only two revisional cases have developed infection 30 days post-operation, one case relates to class III and the other to class IV. In class V there was no infection event; after adjusting to multiple comparisons, infection rate was not different between the BMI groups (p=0.042, FDR>0.999). We identified only 1 adverse event in the class V group, a 43-year-old male patient with a BMI of 63.0 kg/m2 who underwent an uneventful OAGB surgery. He was readmitted one week later due to abdominal pain. Computed tomography of the chest and abdomen was normal, and he was discharged home the following day.
There were 2 deaths within 30 days of surgery (mortality rate: 0.06%). Both cases were in the class III group. One patient developed septic shock due to leak at the gastroenteroanastomosis (GEA). The second event occurred following a leak at the GEA, which developed after successful endoscopic treatment for intra-luminal bleeding.
4. Discussion
In the last several years, the most frequently performed MBS procedure in Israel is OAGB. We aimed to evaluate the perioperative outcomes of primary and revisional OAGB patients with class IV and V obesity relative to class III obesity in a sample of 3,229 consecutive patients. Postoperative patient-reported complications did not differ in level of CDC severity across BMI groups or in the primary vs. revisional cohorts. Mortality was low (n=2; 0.06%) and was limited to patients with class III obesity. Although the class III group was markedly larger, no statistically significant difference in the proportion or severity of overall ≤30-day complications was observed except for the length of operative time, which increased as the BMI class increased.
Prior MBS studies have found that BMI is an independent predictor of operative time [20, 21], and that prolonged operative time is directly correlated with increased MBS complications [22, 23]. Yet, in the current OAGB-specific study, although operative time was higher in each respective BMI class (III, IV, V: 66.5±25.6, 70.5±28.7, and 80.0±34.7 minutes; p= 0.018), this difference did not increase the rates of ≤30-day complications, reoperations, or readmissions.
A survey of 789 MBS surgeons worldwide regarding selection of surgery and management of patients with class IV obesity found that identifying the safest effective procedure for high-BMI patients was a primary challenge [24]. With respect to OAGB, a recent systematic review by Parmar et al. that directly compared OAGB to SG and RYGB in class IV and V obesity concluded that the safety of OAGB was equivalent to that of the other two procedures [15]. Still, the existing evidence conflicts on the topic of perioperative complications in higher-BMI patients after OAGB, SG, and RYGB. Nasser et al. national database analysis comparing perioperative outcomes of high-BMI classes after SG and RYGB found statistically significant differences between groups in complications, readmissions, and mortality but not in terms of reoperations [25]. Gray et al., in a study of perioperative outcomes after robotic SG and RYGB in BMI class III, IV, and V patients, matched in baseline characteristics, and Singhal et al., in a propensity score-matched analysis of high-BMI OAGB, SG, and RYGB patients found no significant differences in intraoperative complications, operative time, postoperative complications, or readmissions between groups [26, 27]. Studies specific to OAGB in comparison to SG in high-BMI patients found comparable rates of early complications [11, 12, 14, 28].
Evidence also varies in reports of non-OAGB MBS procedures that focus on elevated comorbidities in patients with class IV and V obesity. Some studies show a direct correlation between the disease burden and incidence of ≤30-day morbidity and mortality compared to those with class III obesity [28-30]. In the current study, the higher rate of comorbidities in the class V group was not significantly different from that of the class III and IV groups and thus could not be directly compared to other studies.
In the current study, the early OAGB postoperative complication rate inclusive of all BMI categories and both primary and revisional procedures was 3.2-4.3%, with no mortality in the two highest BMI classes. We found a trend toward a higher rate of total early adverse events in the two highest BMI groups, but this did not reach statistical significance (p=0.926). Moreover, this difference was not found in revisional surgery. Long-term, rigorous study designs, with higher representation of class V obesity, are needed to facilitate more precise comparisons of perioperative complications between high-BMI groups.
5. Conclusion
OAGB was a safe MBS procedure in patients with BMI class IV and V without a significantly increased risk of complications compared to patients with class III obesity. These findings were consistent across primary and revisional surgeries. Further investigation of both perioperative and long-term complications is needed in patients with class IV and V obesity undergoing OAGB.
Limitations and Strengths
The main strength of this study is the large sample size. There are several limitations of the study. First, the main outcome of this study, early postoperative complications, were monitored by telephone calls performed 30 days post-surgery. This may result in missing data lack of compliance. However, patients are also instructed to attend the center where the operation was performed in case of any complication during the first 30-day post-surgery. This procedure ensures that there are no complications that could have been missed, even among patients that refused to complete the telephone survey. Next, although the general study sample size is notably large, the BMI groups were unbalanced, and class V group had insufficient power due to its extreme condition. Further, we investigated only early postoperative complications, and long-term adverse were not evaluated. However, outcomes of several large mid- and longer-term OAGB patient series have been published [31-35], but very short-term safety studies are less common. Last but yet important, as surgeons preferred to perform the more difficult cases in public rather than private hospitals, the potential for selection bias may exist. Moreover, all OAGB surgeries that were included in this study were performed by highly experienced bariatric surgeons. Therefore, our results may not be generalizable to OAGB procedures carried out at public hospitals or by less experienced surgeons.
Conflicts of Interest
None.
Acknowledgments
We thank JN Buchwald and TW McGlennon, Medwrite Medical Communications, Maiden Rock, WI, USA for assistance with manuscript development, for which they received a grant.
Statement of Ethics
The study protocol, and all procedures of the study were approved as compliant with the medical center’s ethical standards.
Informed Consent
Informed consent was waived due to the retrospective and anonymous nature of data collected.
Ethical Standards / Human and Animal Rights
The study was approved by the Assuta Medical Centers' ethics committee (43-20-ASMC 13/9/2020).
Assuta Bariatric Surgeons Collaborative
Ahmad Assalia, Subhi Abu Abeid, Igor Dashkovsky, Oleg Dukhno, Dvir Froylich, Shai Meron Eldar, Anya Wexler Feigin, Nissim Geron, Jamal Gazmawi, David Hazzan, Andrei Keidar, Hasan Kais, Ahmad Mahajna, Hussam Madi, Ibrahim Matter, Amnon Ovnat, Mordechai Shimonov, Gideon Sroka, Shimon Sapojnikov, Igor Waksman.
REFERENCES
[1] World Obesity
Federation. World Obesity Atlas 2022. View at: Publisher
Site
[2] World Health
Organization. Obesity and overweight fact sheet, 2021. View at: Publisher
Site
[3] Nasser Sakran,
Shiri Sherf-Dagan, Orit Blumenfeld, et al. “Incidence and Risk Factors for
Mortality Following Bariatric Surgery: a Nationwide Registry Study”. Obes
Surg, vol. 28, no. 9, pp. 2661-2669, 2018. View at: Publisher Site | PubMed
[4] K Hope Wilkinson,
Melissa Helm, Kathleen Lak, et al. “The Risk of Post-operative Complications in
Super-Super Obesity Compared to Super Obesity in Accredited Bariatric Surgery
Centers.” Obes Surg, vol. 29, no. 9, pp. 2964-2971, 2019. View at: Publisher Site | PubMed
[5] Uri Kaplan, Orly
Romano-Zelekha, David Goitein, et al. “Trends in Bariatric Surgery: a 5-Year
Analysis of the Israel National Bariatric Surgery Registry.” Obes Surg,
vol. 30, no. 5, pp. 1761-1767. View at: Publisher Site | PubMed
[6] Manish Parikh, Dan
Eisenberg, Jason Johnson, et al. “American Society for Metabolic and Bariatric
Surgery review of the literature on one-anastomosis gastric bypass.” Surg
Obes Relat Dis, 2018 Aug; vol. 14, no. 8, pp. 1088-1092, 2018. View at: Publisher Site | PubMed
[7] Dimitrios E
Magouliotis, Vasiliki S Tasiopoulou, George Tzovaras “One Anastomosis Gastric
Bypass Versus Roux-en-Y Gastric Bypass for Morbid Obesity: an Updated
Meta-Analysis.” Obes Surg, vol. 29, no. 9, pp. 2721-2730. View at: Publisher Site | PubMed
[8] R Rutledge “The
mini-gastric bypass: experience with the first 1,274 cases.” Obes Surg,
vol. 11, no. 3, pp. 276-280, 2001. View at: Publisher Site | PubMed
[9] Robert Rutledge,
Kuldeepak Kular, Naveen Manchanda “The Mini-Gastric Bypass original technique.”
Int J Surg, vol. 61, pp. 38-41, 2019. View at: Publisher Site | PubMed
[10]
Maurizio
De Luca, Giacomo Piatto, Giovanni Merola, et al. “IFSO Update Position
Statement on One Anastomosis Gastric Bypass (OAGB).” Obes Surg, vol. 31,
no. 7, pp. 3251-3278, 2021. View at: Publisher Site | PubMed
[11]
Vitish
Singla, Sandeep Aggarwal, Bhanu Singh et al. “Outcomes in Super Obese Patients
Undergoing One Anastomosis Gastric Bypass or Laparoscopic Sleeve Gastrectomy.” Obes
Surg, vol. 29, no. 4, pp. 1242-1247, 2019. View at: Publisher Site | PubMed
[12]
Andreas
Plamper, Philipp Lingohr, Jennifer Nadal, et al. “Comparison of mini-gastric
bypass with sleeve gastrectomy in a mainly super-obese patient group: first
results.” Surg Endosc, vol. 31, no. 3, pp. 1156-1162, 2017. View at: Publisher Site | PubMed
[13]
Cesare
Peraglie “Laparoscopic mini-gastric bypass (LMGB) in the super-super obese:
outcomes in 16 patients.” Obes Surg, vol. 18, no. 9, pp. 1126-1129,
2008. View at: Publisher
Site | PubMed
[14]
Brijesh
Madhok, Kamal K Mahawar, Maureen Boyle, et al. “Management of Super-super Obese
Patients: Comparison Between Mini (One Anastomosis) Gastric Bypass and Sleeve
Gastrectomy.” Obes Surg, vol. 26, no. 7, pp. 1646-1649, 2016. View at: Publisher Site | PubMed
[15]
Chetan
D Parmar, Catherine Bryant, Enrique Luque-de-Leon, et al. “One Anastomosis
Gastric Bypass in Morbidly Obese Patients with BMI ≥ 50 kg/m2: a
Systematic Review Comparing It with Roux-En-Y Gastric Bypass and Sleeve
Gastrectomy.” Obes Surg, vol. 29, no. 9, pp. 3039-3046, 2019. View at: Publisher Site | PubMed
[16]
Israel
Center for Disease Control (ICDC) “Israel National Health Interview Survey
INHIS-3, 2013-2015 - selected findings. ICDC, Ministry of Health publication.”
vol. 374, 2017. View at: Publisher
Site
[17]
Eisenman
Y “Israel Central Bureau of Statistics. Selected data on health and way of
life, from the 2017 social survey: weight, dieting, nutrition and eating
habits.” Central Bureau of Statistics, 2018.
[18]
Daniel
Dindo, Nicolas Demartines, Pierre-Alain Clavien “Classification of surgical
complications: a new proposal with evaluation in a cohort of 6336 patients and
results of a survey.” Ann Surg, vol. 240, no. 2, pp. 205-213, 2004. View
at: Publisher
Site | PubMed
[19]
Sakran
N, Sherf-Dagan S, Hod K, et al. “Early outcomes of 6,722 patients following
one anastomosis gastric bypass based on the Assuta Surgery Registry: a retrospective cohort study.” Obes
Surg, In submission, 2023.
[20]
Mei
M Chan, Numan Hamza, Basil J Ammori “Duration of surgery independently
influences risk of venous thromboembolism after laparoscopic bariatric
surgery.” Surg Obes Relat Dis, vol. 9, no. 1, pp. 88-93, 2013. View at: Publisher Site | PubMed
[21]
Michael
L Schwartz, Raymond L Drew, Marilyn Chazin-Caldie “Laparoscopic Roux-en-Y
gastric bypass: preoperative determinants of prolonged operative times,
conversion to open gastric bypasses, and postoperative complications.” Obes
Surg, vol. 13, no. 5, pp. 734-738, 2003. View at: Publisher Site | PubMed
[22]
Colette
S Inaba, Christina Y Koh, Sarath Sujatha-Bhaskar, et al. “Operative time as a
marker of quality in bariatric surgery.” Surg Obes Relat Dis, vol. 15,
no. 7, pp. 1113-1120, 2019. View at: Publisher Site | PubMed
[23]
Joseph
A Sanford, Bassam Kadry, Jay B Brodsky “Bariatric surgery operating room
time--size matters.” Obes Surg, vol. 25, no. 6, pp. 1078-1085, 2015.
View at: Publisher
Site | PubMed
[24]
Mohammad
Kermansaravi, Panagiotis Lainas, Shahab Shahabi Shahmiri, et al. “The first
survey addressing patients with BMI over 50: A survey of 789 bariatric
surgeons.” Surg Endosc, vol. 36, no. 8, pp. pp. 6170-6180, 2022. View
at: Publisher
Site | PubMed
[25]
Hassan
Nasser, Tommy Ivanics, Shravan Leonard-Murali, et al. “Perioperative outcomes
of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in super-obese
and super-super-obese patients: a national database analysis.” Surg Obes
Relat Dis, vol. 15, no. 10, pp. 1696-1703, 2019. View at: Publisher Site | PubMed
[26]
Katherine
D Gray, Alfons Pomp, Gregory Dakin, et al. “Perioperative outcomes and
anesthetic considerations of robotic bariatric surgery in a propensity-matched
cohort of super obese and super-super obese patients.” Surg Endosc, vol.
32, no. 12, pp. 4867-4873, 2018. View at: Publisher Site | PubMed
[27]
Rishi
Singhal, Victor Roth Cardoso, Tom Wiggins, et al. “30-day morbidity and
mortality of sleeve gastrectomy, Roux-en-Y gastric bypass and one anastomosis
gastric bypass: a propensity score-matched analysis of the GENEVA data.” Int
J Obes (Lond), vol. 46, no. 4, pp. 750-57, 2022. View at: Publisher Site | PubMed
[28]
Sophia
M-T Schmitz, Patrick H Alizai, Andreas Kroh, et al. “Clinical outcomes after
one anastomosis gastric bypass versus sleeve gastrectomy in super-super-obese
patients.” Surg Endosc, vol. 36, no. 6, pp. 4401-4407, 2002. View at: Publisher Site | PubMed
[29]
S
Paeratakul, J C Lovejoy, D H Ryan, G A Bray “The relation of gender, race and
socioeconomic status to obesity and obesity comorbidities in a sample of US
adults.” Int J Obes Relat Metab Disord, vol. 26, no. 9, pp. 1205-1210,
2002. View at: Publisher
Site | PubMed
[30]
John
Cawley, Matthew J Sweeney, Marina Kurian, et al. “Predicting complications
after bariatric surgery using obesity-related comorbidities.” Obes Surg,
vol. 17, no. 11, pp. 1451-1456, 2007. View at: Publisher Site | PubMed
[31]
M
Musella, A Susa, F Greco, et al. “The laparoscopic mini-gastric bypass: the
Italian experience: outcomes from 974 consecutive cases in a multicenter
review.” Surg Endosc, vol. 28, no. 1, pp. 156-163, 2014. View at: Publisher Site | PubMed
[32]
Jean
Marc Chevallier, Gustavo A Arman, Martino Guenzi, et al. “One thousand single
anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year
period: outcomes show few complications and good efficacy.” Obes Surg,
vol. 25, no. 6, pp. 951-958, 2015. View at: Publisher Site | PubMed
[33]
Osama
Taha, Mahmoud Abdelaal, Mohamed Abozeid, et al. “Outcomes of Omega Loop Gastric
Bypass, 6-Years Experience of 1520 Cases.” Obes Surg, vol. 27, no. 8,
pp. 1952-1960, 2017. View at: Publisher Site | PubMed
[34] Yonatan Lessing, Niv Pencovich, Marian Khatib, et al. “One-Anastomosis Gastric Bypass: First 407 Patients in 1 year.” Obes Surg, vol. 27, no. 10, pp. 2583-2589, 2017. View at: Publisher Site | PubMed
[35] Miguel A Carbajo, Enrique Luque-de-León, José M Jiménez, et al. “Laparoscopic One-Anastomosis Gastric Bypass: Technique, Results, and Long-Term Follow-Up in 1200 Patients.” Obes Surg, vol. 27, no. 5, pp. 1153-1167, 2017. View at: Publisher Site | PubMed
