Received: Fri 11, Aug 2023
Accepted: Thu 31, Aug 2023
Abstract
Introduction: Primary bone tumours of the distal tibia are rare, and amputation was the treatment of choice in the past. Custom-made endoprosthesis reconstruction of the distal tibia has become a viable option in selected patients as a form of limb salvage for better function and cosmesis. It provides similar functional outcomes to below-knee amputation with a below-knee prosthesis. We investigated our cases' reliability, functional and oncological outcomes with distal tibia endoprosthesis reconstruction done for aggressive bone tumours. Methods: This is a retrospective review of 10 patients who underwent distal tibia endoprosthesis reconstruction at our centre from December 2007 to December 2021. This study aims to determine the clinical, oncological and functional outcomes using the Musculoskeletal Tumor Society (MSTS) Score and Toronto Extremity Salvage Score (TESS) and the survivorship of these implants. Results: The mean age of our patients is 29 years (range 12 to 53), with 60% males. The mean follow-up was 39.3 months (range 12 to 112). 50% of our cases were giant cell tumour of bone, 20% were osteosarcoma, and 10% were ewing’s, PVNS and pleomorphic sarcoma of the bone, respectively. Two patients passed away from advanced disease, where one was associated with local recurrence. The other case with local recurrence developed an acute infection post-resection of the recurrence. The infection resolved after repeated debridement and free muscle transfer. One patient had septic loosening of the implant and underwent below-knee amputation. Two-thirds of the patients remained disease free. The mean MSTS score for the 9 patients during their last follow-up is 87.8% (range 73.3 to 100). The mean TESS score for the 6 patients during their last follow-up is 84.4% (range 69.2 to 100). Conclusion: Our study concludes that distal tibia endoprosthesis reconstruction is a reliable treatment option for aggressive bone tumours and provides good function outcomes.
1. Introduction
Tumours of the distal tibia are considered rare [1-3]. Both benign aggressive lesions of the bone and primary bone malignancies such as giant cell Tumors and osteosarcoma commonly involve the distal femur and proximal tibia, rarely affecting the distal tibia and ankle [4-9]. The tibia is the second most common site of all osteosarcoma (19%), and only 20% of these tumours occur in the distal tibia [7, 10]. Whereas for giant cell tumours of the bone, only less than 4% affects the distal tibia [6, 11]. According to current available studies on primary bone tumours of the distal tibia, the most common pathologic diagnosis is osteosarcoma (75.1%), followed by ewing’s sarcoma (7.4%) and giant cell tumour of the bone (6.8%) [12].
Amputation was the standard treatment method for primary bone tumours affecting the distal lower limb for the past decade [13-15]. However, due to advancements in surgical techniques, modern imaging modalities, and better efficacy chemotherapy regimens, limb salvage has become the current mainstay of treatment for distal lower limb tumours, proven by multiple studies [9, 11, 13, 16-20]. Among various types of limb salvage surgery, endoprosthesis reconstruction (EPR) of the distal tibia remained controversial due to its rarity and the small sample sizes of most studies.
We aim to study the outcome of patients who underwent distal tibia endoprosthesis replacement in our centre to determine the reliability of this treatment option. This retrospective review describes the clinical, oncological and functional outcomes compared to published data.
2. Materials and Methods
2.1. Patients
Between 2007 to 2021, we reviewed all our records of patients who underwent endoprosthesis replacements; we identified 10 patients with primary bone tumours of the distal tibia who underwent distal tibia endoprosthesis reconstruction following resection of an aggressive bone tumour. These patients were identified by a retrospective review from a prospectively maintained database. The medical records, imaging, pathology data and outcomes were reviewed.
2.2. Prosthesis
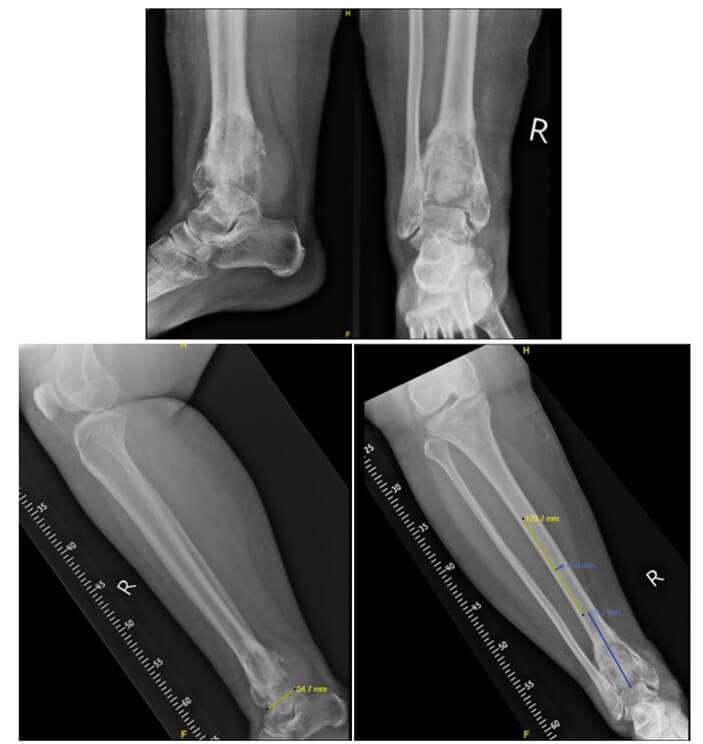
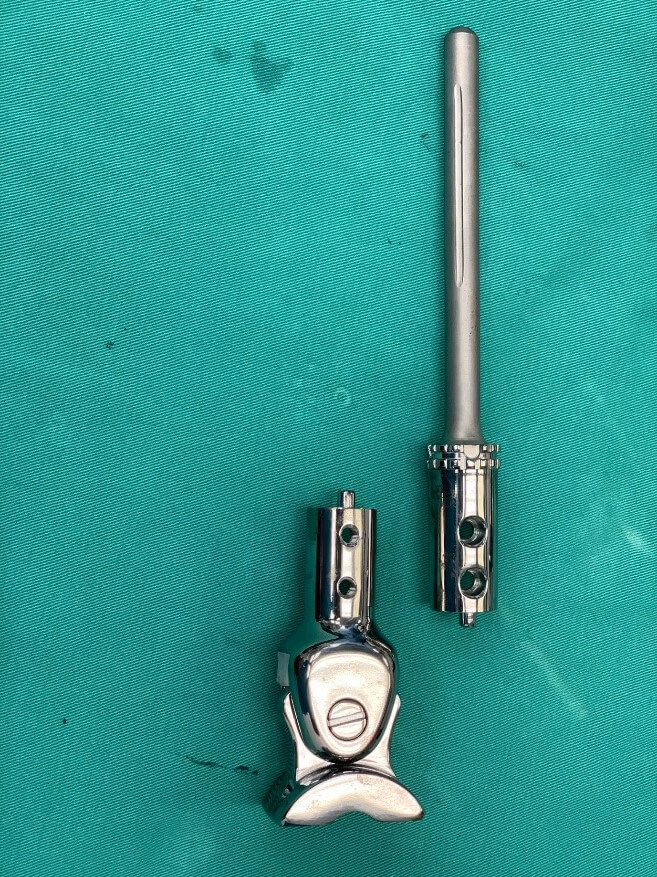
Our prosthesis is custom-made from Eagle Osteon Technologies, Chennai, India. Measured roentgenograms are used to determine the size of the resection segment, the size and length of the tibial stem and the dimensions of the talar articulating segment (Figure 1). Based on these measurements, a stretch is drawn by the engineers (Figure 2). The prosthesis is manufactured from titanium alloy and consists of two components locked together by screws and a central peg (Figure 3). The distal articulating segment is a saddle joint that sits on the dome of the talus and is secured with cementation and screws (Figure 4). The saddle joint of the prosthesis consists of a bar lined with a polyethene liner that articulates with an oblong hole at the superior aspect of the talus end of the prosthesis. The proximal component has a cylindrical stem cemented into the proximal end of the tibia. The articular surfaces are lined by ultra-high-molecular-weight polyethene. The movement at the ankle is not a hinge movement but rather a saddle joint movement to better mimic the mechanics of a normal ankle joint.
2.3. Operative Criteria
All the patients identified for this procedure fulfilled the following criteria for limb salvage surgery:
i) No neurovascular bundle (anterior tibial artery, posterior tibial artery and posterior tibial nerve).
ii) No skip lesions within the same tibia.
iii) No ipsilateral talar or Calcaneum involvement.
2.4. Operative Technique
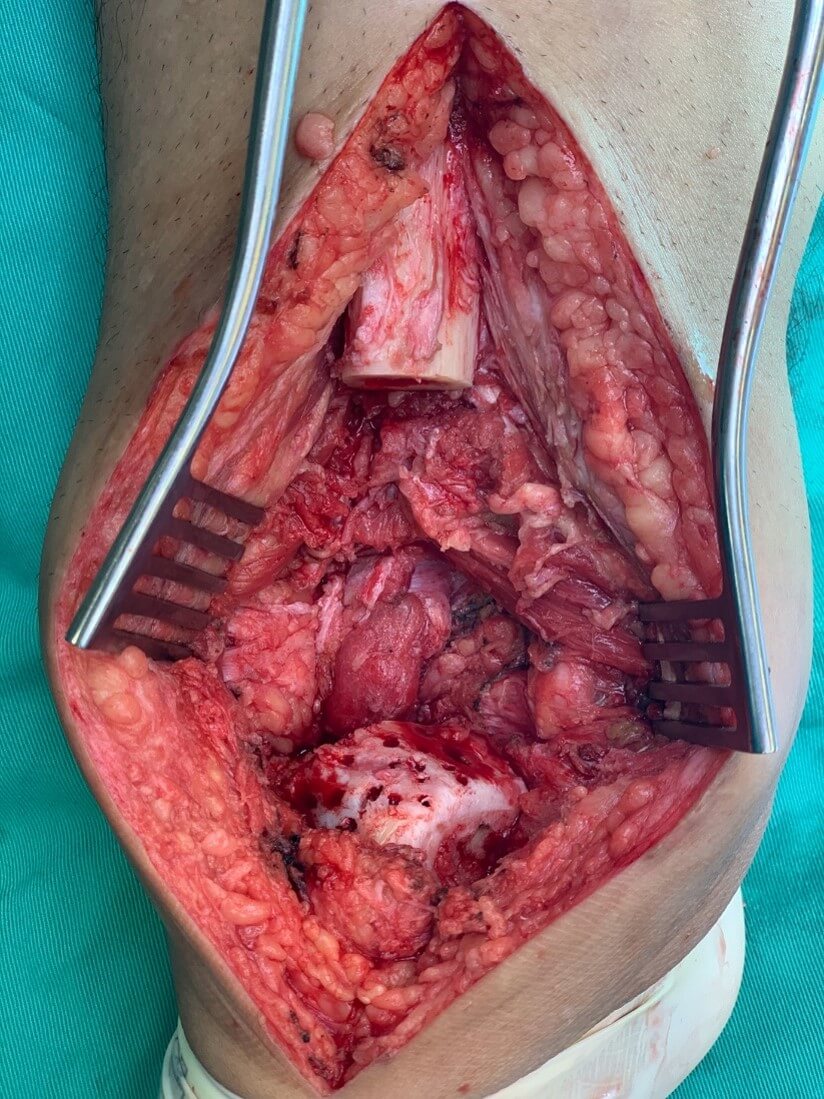
The operative approach is an anterior lateral incision. The biopsy tract is incorporated within the incision and excised with the tumour. Tumour dissection is carried out with the intent of obtaining wide resection margins. The anterior or posterior tibia arteries or both arteries are preserved with the posterior tibia nerve during resection. We usually try to preserve the superficial and deep peroneal nerve branches, but often they are removed with the tumour. The proximal tibial bone cuts are carried out about 2 to 3 cm from the proximal margin of the tumour. These cuts are planned and incorporated into the design of the implant. The tumour is removed at the ankle joint, separating the talus from the distal tibia fibula joint without breaching the distal tibia articular cartilage. The distal portion of the fibula with the distal tibia fibula joint is usually removed to prevent recurrence due to tumour infiltration of this joint. Haemostasis is secured once the tumour is removed, and the wound is washed generously with a lavage gun.
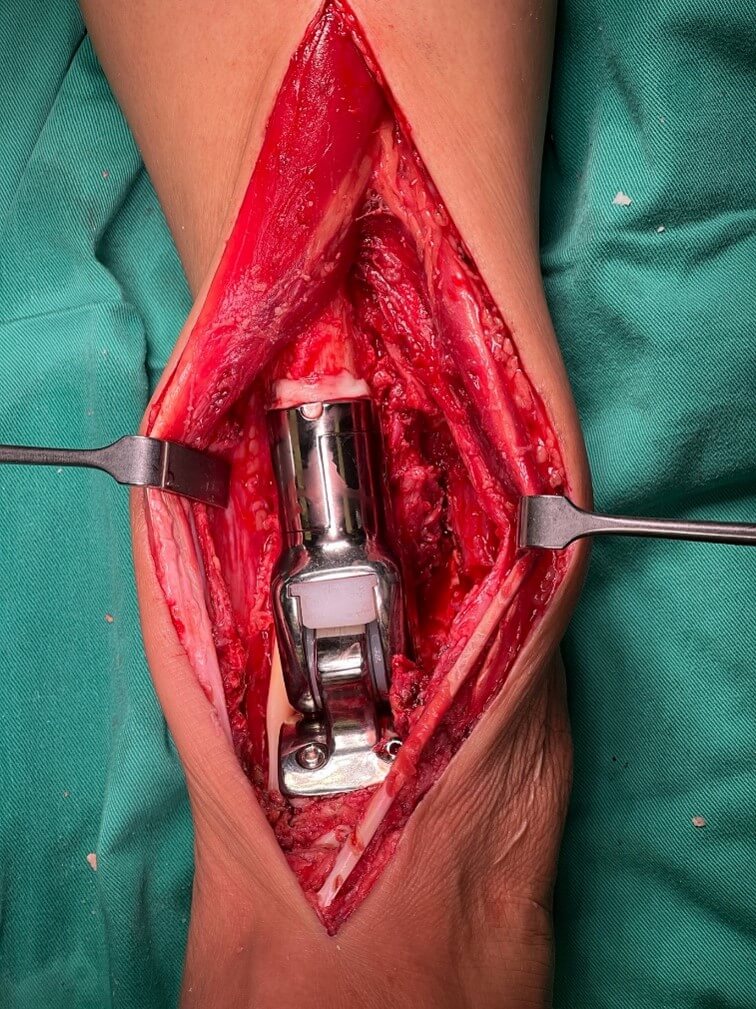
Next, the cartilage over the talar dome is shaved off with a power saw, the talar dome is fashioned to fit the cup end of the prosthesis. Multiple drill holes are made over the talus dome for the anchorage of the cement (Figure 5). This component is cemented onto the talus and secured with threaded cancellous screws. The proximal remaining end of the tibia is reamed to a size about 2 mm bigger than the stem diameter, and the stem is cemented to achieve fixation. A cement plug is often not used as the implant's stem is designed long enough to accommodate the remaining bone segment. The two components of the implant are subsequently reduced and locked together by the peg and secured by two screws (Figure 6).
2.5. Evaluation
Clinical data were reviewed retrospectively from all patients’ medical records and radiological imaging. Functional Assessments were made during each follow-up visit based on the Musculoskeletal Tumor Society Functional Assessment Society (MSTS) criteria in all patients. The Toronto Extremity Salvage Score (TESS) collection was only implemented in 2015. The latest MSTS and TESS score is reported.
3. Results
The results are presented in (Table 1). Ten patients with a mean age of 29 years (12 to 53 years) were treated for aggressive bone tumours, consisting of 6 males and 4 females. The mean follow-up for all cases was 39.3 months (12 to 112 months). Our study comprises five patients with giant cell tumours (GCT), one ewing’s sarcoma, two osteosarcoma (OS), one pleomorphic sarcoma of the bone and one pigmented villonodular synovitis (PVNS). One of the patients with GCT had stable lung metastasis at diagnosis, and the lung disease remained static. One patient was lost to follow-up after surgery as she was a foreigner and returned to her own country and was followed up there. Two patients (one with osteosarcoma and one with ewings) passed away from advanced disease. Two patients developed local recurrence, one was a case of GCT and another OS. Two patients (one with OS and one with ewings) passed away due to advanced disease at 12 and 18 months, respectively. Two patients (20%) in this series developed significant infective complications. The first is the lady with GCT who had two episodes of soft tissue local recurrence and underwent resection at 13 months and 14.5 months post-EPR, respectively. This patient developed a wound breakdown post-resection and an implant-related infection two months after the second surgery. Her infection resolved after repeated debridement and free muscle transfer; she was free of the disease and infection in her latest follow-up.
TABLE 1: Shows the
demographics of our patients.
|
Age
(years) |
Gender |
Diagnosis |
Metastasis
at diagnosis |
Follow-up
(months) |
Oncological
Status |
Complications |
MSTS
(%) |
TESS
(%) |
|
|
1 |
25 |
Male |
Giant Cell Tumor |
No |
112 |
Free of disease |
No |
86.7 |
75.0 |
|
2 |
20 |
Female |
Giant Cell Tumor |
Yes (Lung) |
22 |
Free of disease |
Wound breakdown, Septic loosening
and Transtibial amputation |
96.7 |
Nil |
|
3 |
31 |
Male |
Giant Cell Tumor |
No |
82 |
Free of disease |
No |
93.3 |
92.2 |
|
4 |
27 |
Male |
Giant Cell Tumor |
No |
32 |
Free of disease |
No |
86.7 |
Nil |
|
5 |
20 |
Male |
Osteosarcoma |
No |
12 |
Died of disease: Lung metastasis |
Local recurrence |
90.0 |
Nil |
|
6 |
53 |
Female |
Pleomorphic Sarcoma |
No |
Loss to follow-up |
Nil |
Nil |
Nil |
Nil |
|
7 |
19 |
Male |
Ewing's Sarcoma |
No |
18 |
Died of disease: Brain and lung
metastasis |
No |
73.3 |
69.2 |
|
8 |
12 |
Male |
Osteosarcoma |
No |
42 |
Free of disease |
No |
100.0 |
100.0 |
|
9 |
34 |
Female |
Giant Cell Tumor |
No |
19 |
Free of disease |
Infection, Wound breakdown and
Local recurrence |
90.0 |
95.0 |
|
10 |
52 |
Female |
Pigmented Villonodular Synovitis |
No |
15 |
Free of disease |
No |
73.3 |
75.0 |
The other is the patient with the GCT with stable lung metastasis developed septic implant loosening. She underwent stage 1 of a planned 2-stage revision but opted for a below-knee amputation after a stage 1 revision procedure at 21 months post-EPR. She had MSTS functional score of 96.7% before the septic loosening. Half of the patients had no complications and remained disease-free post-EPR. The mean MSTS score for the 9 patients during their last follow-up is 87.8% ± 9.3 (range 73.3 to 100). The mean TESS score for the 6 patients during their last follow-up is 84.4% ± 12.8 (range 69.2 to 100). All of the patients were able to walk without support during their last follow-up.
4. Discussion
In this retrospective review, the patient with osteosarcoma who underwent EPR has a worse prognosis than those with other diseases. The young male patient died 12 months post-EPR due to extensive metastases to the brain and lungs. This patient showed a poor response to chemotherapy, with only 10% necrosis in the resected specimen after neoadjuvant chemotherapy. Our data reflects that most of our patients remained free of disease (70%), and 85.7% of them could preserve the lower limb after the resection of an aggressive bone tumour affecting the distal tibia (Table 1). These patients' mean MSTS score for the latest follow-up is 88.3%.
Various surgical options other than the traditional transtibial amputation for the aggressive tumours of the distal tibia have been explored in recent decades, including endoprosthesis replacement, autograft, allograft, and distraction osteogenesis [11]. However, insufficient studies are still comparing distal tibia endoprosthesis reconstruction and transtibial amputation with prosthesis to determine the better treatment option. Traditionally, compared with amputation of the distal lower limb, endoprosthesis reconstruction would provide a better psychosocial outcome due to the unneglectable cosmetic difference and the preservation of a normal-looking limb. However, assessing the quality of life is difficult as many variables have to be considered, including social well-being, marital status and employment [22]. Mavrogenis et al. [9] have proven that limb salvage surgery provided similar survival with amputation. Although local recurrence and complications were more common in limb salvage surgery, it is a risk worth taking for a selected group of patients to achieve better cosmesis and functionality.
Among these options for limb salvage reconstruction surgeries, arthrodesis was considered the best choice, as it provides excellent stability at the ankle joint and lesser complications than prosthetic implantation [11]. Zhao et al. [12] suggested biological reconstruction as the first choice considering its better postoperative functional outcome and lesser major complications. However, biological reconstruction had complications such as fracture, non-union, and deep infection [12, 19, 20, 23-26]. All forms of limb salvage surgeries possess the risk of local recurrence.
Table 2 compares the results of the existing studies on the outcome of endoprosthesis reconstructions with our study. The patients ranged from 5 to 14. In total, are 40 cases reported, excluding our series, Yang et al. [15] review is an update of a similar cohort of patients as reported by Abudu et al. [13]. We have the highest functional scores among the published series, with complication rates of 20% of local recurrence, infection and amputation each.
TABLE 2: Shows a
comparison between published series on EPR.
|
No. |
Study |
No. of patients |
Follow-up (months) |
Local recurrence |
Metastases |
Infection |
Amputation/Revision |
Functional outcome (%) |
|
1 |
Abudu et
al. [10] |
5 |
55 |
1 |
1 |
1 |
0 |
MSTS:
64 |
|
2 |
Lee et
al. [4] |
6 |
65 |
0 |
0 |
1 |
0 |
ISOLS:
80 |
|
3 |
Natarajan
et al. [5] |
6 |
40 |
2 |
0 |
1 |
3 |
MSTS:
80 |
|
4 |
Shekkeris
et al. [26] |
6 |
115 |
0 |
0 |
2 |
2 |
MSTS:
70 TESS:
71 |
|
5 |
Yang et
al. [15] |
8 |
77 |
2 |
3 |
2 |
1 |
MSTS:
66 |
|
6 |
Raciborska
et al. [27] |
14 |
20 |
2 |
3 |
1 |
0 |
MSTS:
73 |
|
7 |
Our series |
10 |
39 |
2 |
2 |
2 |
2 |
MSTS:
88 TESS:
84 |
Zhao et al. [12], in their systematic review of surgical treatment of primary distal tibia tumours, compared the various forms of reconstruction, including amputation. The highest functional scores were reported for cases with distraction osteogenesis (MSTS 91%), followed by autograft (MSTS 80.2%). Allograft reconstruction, EPR and amputation had a functional score (MSTS) of 74.3%, 72.2% and 70.9%. The complications (excluding local recurrence) reported in the pooled data were the highest for the EPR (35.1%), with infection prevailing (16.2%), followed by allograft reconstruction (35%), mainly due to fracture (15.1%). Distraction osteogenesis had a complication rate of 30% (mainly due to non-union 20%), and autograft had the lowest complication rate of 16.9% (mainly due to fracture 7%). Amongst all the forms of reconstruction, ankle movement was preserved only in those who underwent EPR.
Distal tibia EPR enables early mobilisation, weight bearing, immediate stability, and better cosmesis than other reconstructions. Our patients can achieve excellent ankle stability at their latest follow-up, not requiring any support during ambulation. Furthermore, no mechanical failures or talar collapses are noted in our series.
We recognise the limitation of our study. It is a retrospective single-institution review with limited numbers. However, distal tibial tumours are rare, making obtaining larger patient numbers difficult.
5. Conclusion
We believe that endoprosthesis reconstruction of the distal tibia is a reliable alternative option as it provides good functional outcomes, and the complication rates are acceptable compared to other options of reconstruction, including amputation. We prefer this method of reconstruction in patients in whom limb salvage surgery is possible. Amputation can be reserved as the last resort if complications arise.
Conflicts of Interest
None.
Author Contributions
Khaw Kar Wei: Collected the data and wrote the draft of the manuscript. Vivek Ajit Singh: Conceptualization, Supervision and Review and editing. Nor Faissal Yasin, Ling Xiu Wen and Azura Mansor: Project administration, Review and Editing.
REFERENCES
[1] Stefan S Bielack, Beate
Kempf-Bielack, Günter Delling, et al. “Prognostic factors in high-grade
osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteo- sarcoma study group protocols.” J Clin
Oncol, vol. 20, no. 3, pp. 776-790, 2002. View at: Publisher Site | PubMed
[2] Walid Ebeid, Sherif Amin, Amr
Abdelmegid, et al. “Reconstruction of distal tibial defects following resection
of malignant tumours by pedicled vascularised fibular grafts.” Acta Orthop
Belg, vol.73, no. 3, pp 354-359, 2007. View at: PubMed
[3] Andreas F Mavrogenis, Caterina
Novella Abati, Carlo Romagnoli, et al. “Similar survival but better function
for patients after limb salvage versus amputation for distal tibia osteo- sarcoma.”
Clin Orthop Relat Res, vol. 470, no. 6, pp. 1735-1748, 2012. View at: Publisher Site | PubMed
[4] S H Lee, H S Kim, Y B Park, et al.
“Prosthetic reconstruction for tumours of the distal tibia and fibula.” J
Bone Joint Surg Br, vol. 81, no. 5, pp. 803-807, 1999. View at: Publisher Site | PubMed
[5] M V Natarajan, K Annamalai, S
Williams, et al. “Limb salvage in distal tibial osteosarcoma using a custom
mega prosthesis.” Int Orthop, vol. 24, no. 5, pp. 282-284, 2000. View
at: Publisher Site | PubMed
[6] W I Faisham, W Zulmi, A S Halim, et
al. “Aggressive giant cell tumour of bone.” Singapore Med J, vol. 47, no. 8,
pp. 679-683, 2006. View at: PubMed
[7] Giulia Ottaviani, Norman Jaffe “The
epidemiology of osteosarcoma.” Cancer Treat Res, vol. 152, pp. 3-13,
2009. View at: Publisher
Site | PubMed
[8] Marta Karpik “Giant Cell Tumor (tumor
gigantocellularis, osteoclastoma) - epidemiology, diagnosis, treatment.” Ortop
Traumatol Rehabil, vol. 12, no. 3, pp. 207-215, 2010. View at: PubMed
[9] Andreas F Mavrogenis, Caterina
Novella Abati, Carlo Romagnoli, et al. “Similar survival but better function
for patients after limb salvage versus amputation for distal tibia
osteosarcoma.” Clin Orthop Relat Res, vol. 470, no. 6, pp. 1735-1748,
2012. View at: Publisher
Site | PubMed
[10] Trisha Zeytoonjian, Henry J Mankin,
Mark C Gebhardt, et al. “Distal lower extremity sarcomas: frequency of
occurrence and patient survival rate.” Foot Ankle Int, vol. 25, no. 5,
pp. 325-330, 2004. View at: Publisher
Site | PubMed
[11] Vivek Ajit Singh, Norzatulsyima
Nasirudin, Michael Bernatt “Endoprosthetic reconstruction for giant cell tumors
of the distal tibia: a short term review.” Asia Pac J Clin Oncol, vol.
9, no. 2, pp. 182-189, 2013. View at: Publisher Site | PubMed
[12] Zhiqing Zhao, Taiqiang Yan 1, Wei
Guo, et al. “Surgical options and reconstruction strategies for primary bone
tumors of distal tibia: A systematic review of complications and functional
outcome.” J Bone Oncol, vol. 14, pp. 100209, 2018. View at: Publisher Site | PubMed
[13] A Abudu, R J Grimer, R M Tillman, et
al. “Endoprosthetic replacement of the distal tibia and ankle joint for
aggressive bone tumours.” Int Orthop, vol. 23, no. 5, pp. 291-294, 1999.
View at: Publisher
Site | PubMed
[14] M Laitinen, J Hardes, H Ahrens, et
al. “Treatment of primary malignant bone tumours of the distal tibia.” Int
Orthop, vol. 29, no. 4, pp. 255-259, 2005. View at: Publisher Site | PubMed
[15] P Yang, S Evans, Z Khan, et al.
“Reconstruction of the distal tibia following resection of aggressive bone tumours
using a custom-made megaprosthesis.” J Orthop, vol. 14, no. 3, pp.
406-409, 2017. View at: Publisher
Site | PubMed
[16] M A Simon, M A Aschliman, N Thomas,
et al. “Limb-salvage treatment versus amputation for osteosarcoma of the distal
end of the femur.” J Bone Joint Surg Am, vol. 68, no. 9, pp. 1331-1337,
1986. View at: PubMed
[17] B T Rougraff, M A Simon, J S Kneisl,
et al. “Limb salvage compared with amputation for osteosarcoma of the distal
end of the femur. A long-term oncological, functional, and quality-of-life
study.” J Bone Joint Surg Am, vol. 76, no. 5, pp. 649-656, 1994. View
at: Publisher
Site | PubMed
[18] Achmad Fauzi Kamal, Heru Widyawarman,
Kurniadi Husodo, et al. “Clinical Outcome and Survival of Osteosarcoma Patients
in Cipto Mangunkusumo Hospital: Limb Salvage Surgery versus Amputation.” Acta
Med Indones, vol. 48, no. 3, pp. 175-183, 2016. View at: PubMed
[19] David R Moore, Jennifer L Halpern,
Herbert S Schwartz “Allograft ankle arthrodesis: a limb salvage technique for
distal tibial tumors.” Clin Orthop Relat Res, vol. 440, pp. 213-221,
2005. View at: Publisher
Site | PubMed
[20] Domenico Andrea Campanacci, Guido
Scoccianti, Giovanni Beltrami, et al. “Ankle arthrodesis with bone graft after
distal tibia resection for bone tumors.” Foot Ankle Int, vol. 29, no.
10, pp. 1031-1037, 2008. View at: Publisher Site | PubMed
[21] Giulia Ottaviani, Rhonda S Robert,
Winston W Huh, et al. “Functional, psychosocial and professional outcomes in
long-term survivors of lower-extremity osteosarcomas: amputation versus limb
salvage.” Cancer Treat Res, vol. 152, pp. 421-436, 2009. View at: Publisher Site | PubMed
[22] S Shalaby, H Shalaby, A Bassiony
“Limb salvage for osteosarcoma of the distal tibia with resection arthrodesis,
autogenous fibular graft and Ilizarov external fixator.” J Bone Joint Surg
Br, vol. 88, no. 12, pp. 1642-1646, 2006. View at: Publisher Site | PubMed
[23] Dae-Geun Jeon, Min Suk Kim, Wan
Hyeong Cho, et al. “Reconstruction with pasteurized autograft for distal tibial
tumor.” Arch Orthop Trauma Surg, vol. 128, no. 2, pp. 159-165, 2008.
View at: Publisher Site | PubMed
[24] Rui Niimi, Akihiko Matsumine,
Katsuyuki Kusuzaki, et al. “Usefulness of limb salvage surgery for bone and
soft tissue sarcomas of the distal lower leg.” J Cancer Res Clin Oncol,
vol. 134, no. 10, pp. 1087-1095, 2008. View at: Publisher Site | PubMed
[25] Chunlin Zhang, Bingfang Zeng, Kunpeng
Zhu, et al. “Limb salvage for malignant bone tumours of distal tibia with dual
ipsilateral vascularized autogenous fibular graft in a trapezoid-shaped array
with ankle arthrodesis and preserving subtalar joint.” Foot Ankle Surg,
vol. 25, no. 3, pp. 278-285, 2019. View at: Publisher Site | PubMed
[26] A S Shekkeris, S A Hanna, M D Sewell, et al. “Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours.” J Bone Joint Surg Br, vol. 91, no. 10, pp. 1378-1382, 2009. View at: Publisher Site | PubMed
[27] Anna Raciborska, Katarzyna Bilska, Iwona Malesza, et al. “Distal Tibial Reconstruction in the Management of Primary Bone Tumors in Children and Adolescents.” Foot Ankle Int, vol. 42, no. 11, pp. 1447-1453, 2021. View at: Publisher Site | PubMed
